Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 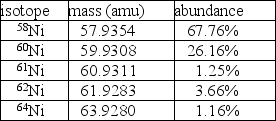 A.Using the data in the table, calculate the average atomic mass for nickel.
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
Definitions:
Examination
An assessment intended to measure a test-taker's knowledge, skill, aptitude, physical fitness, or classification in many other topics.
Functional Teams
Groups of individuals who are organized based on the specific functions they perform within an organization, such as marketing or finance.
Project
A temporary endeavor undertaken to create a unique product, service, or result, with a defined beginning and end.
Procurement Team
A group of individuals within an organization responsible for acquiring goods and services needed for business operations.
Q14: Find the heat absorbed from the
Q16: The first step in the Ostwald
Q28: For the changes made to a dataset
Q28: A ground-state atom of vanadium has _
Q40: Calculate the volume occupied by 25.2 g
Q44: Express the number 26.7 in scientific notation.<br>A)2.67
Q56: Based on the solubility rules, which one
Q64: How many moles of phosphine (PH<sub>3
Q83: Americans combined drive about 4.0 *10<sup>9</sup> miles
Q112: What is the chemical formula of the