Calculate the standard enthalpy of formation of liquid methanol, CH3OH(l) , using the following information: 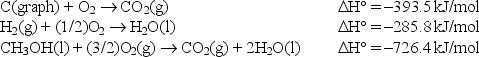
Definitions:
Perfectly Competitive
characterizes a market structure where there are many buyers and sellers, all selling homogeneous products, with no single participant able to influence the market price of the product.
Conditions
The circumstances or factors affecting the way in which people live or work, especially with regard to their safety or well-being.
Investment Capital
Funds invested in a project, company, or any economic endeavor with the expectation of generating a future return.
Efficient Markets
A financial market theory suggesting that asset prices fully reflect all available information, making it impossible to consistently achieve higher returns than the overall market.
Q4: Which one of the following molecules has
Q4: The molecules of different samples of an
Q45: Which one of these polar covalent bonds
Q65: Which element will display an unusually large
Q81: What is the molarity of a solution
Q88: Predict the geometry around the central atom
Q98: According to VSEPR theory, which one of
Q113: For which of these species does the
Q118: Which of the following is an
Q152: Which choice gives the correct oxidation numbers