At 25°C, the following heats of reaction are known: 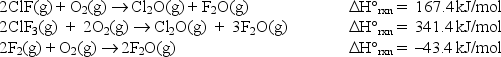
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
Definitions:
Profit-Maximizing
A strategy or point at which a firm reaches the maximum possible profit with its current resources and constraints.
Fixed Cost
Expenses that do not change with the level of output or sales, such as rent or salaries.
Variable Cost
Expenses that change in proportion to the level of production or sales activities of a company, such as raw materials and direct labor costs.
Producer Surplus
The difference between what producers are willing to accept for a good versus what they actually receive, typically represented by an area on a graph.
Q22: Which of the following elements has the
Q51: Calculate the standard enthalpy change for
Q51: Which one of the following does not
Q54: Batteries in our cars generate electricity
Q66: Give the number of lone pairs around
Q82: Which of the following elements behaves chemically
Q103: Write a Lewis structure for the chlorate
Q109: Predict the geometry and polarity of the
Q122: What element is reduced in the
Q153: Identify the element being reduced in