Given the following H° values,
H2(g)+ 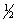 O2(g) H2O(l) H°f = -285.8 kJ/mol
O2(g) H2O(l) H°f = -285.8 kJ/mol
H2O2(l) H2(g)+ O2(g) H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 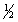 O2(g),
O2(g),
Definitions:
Browser History
A record of web pages a user has visited, stored by a web browser.
Asynchronous Communication
A mode of communication where the exchange of messages occurs without requiring both parties to be simultaneously engaged.
Audio Content
Material or information presented in an audible format, such as music, podcasts, or audiobooks.
B2B Commerce
Business-to-Business commerce, wherein transactions are conducted between companies rather than between companies and consumers.
Q16: Use bond energies to estimate the enthalpy
Q17: Which ground-state atom has an electron configuration
Q37: Which one of the following is not
Q38: A glass containing 200.g of H<sub>2</sub>O at
Q47: Identify the following compounds as a strong
Q48: The total heat of hydration of 1
Q55: How many grams of sulfur are there
Q105: At 25°C, the standard enthalpy of
Q122: What element is reduced in the
Q137: A 0.9182 g sample of CaBr<sub>2</sub> is