Calculate the standard enthalpy of formation of liquid methanol, CH3OH(l) , using the following information: 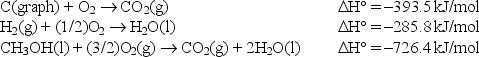
Definitions:
AVC
Average Variable Cost, which is calculated by dividing the variable costs by the quantity of output produced.
Economic Loss
A decrease in financial wealth, including lost opportunity, costs, inefficiencies, or expenditures that exceed the benefits.
Fixed Cost
Costs that do not vary with the level of output or operations, such as rent, salaries, and insurance premiums.
Economic Loss
The negative difference between a company's revenues and its expenses, including opportunity costs.
Q1: Balance the equation below using the
Q22: Ibuprofen is used as an analgesic for
Q32: What is the formal charge on sulfur
Q59: Which one of the following is most
Q67: For a substance that remains a gas
Q75: Write the formula for the acid formed
Q82: The pressure of a gas sample was
Q90: Based on the solubility rules, which of
Q142: A mass spectrometer works by ionizing
Q154: During a titration the following data were