The bond enthalpy of the Br-Cl bond is equal to H° for the reaction BrCl(g) Br(g) + Cl(g) .
Use the following data to find the bond enthalpy of the Br-Cl bond. 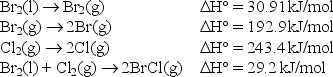
Definitions:
Array
A data structure that stores a fixed-size sequential collection of elements of the same type, allowing multiple items to be stored under one variable name.
Char[][]
Represents a two-dimensional array of characters in languages like Java, used for storing matrices of characters.
Rows
A horizontal group of cells in a table or a spreadsheet, or a horizontal line of data in a database.
Method Definition
The part of a program that specifies the operations that can be performed on or by an object, and the actions taken when the method is invoked.
Q24: What is T in the table below?
Q42: Calculate the standard enthalpy change for
Q58: Which one of the following is
Q73: Identify the element being oxidized in
Q89: Consider the element with the electron configuration
Q102: At body temperature 2,404 joules of energy
Q111: Which of the following species has the
Q114: Use VSEPR theory to explain why the
Q142: Based on the solubility rules, which of
Q149: The percent composition by mass of a