Given the following H° values,
H2(g)+ 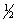 O2(g) H2O(l) H°f = -285.8 kJ/mol
O2(g) H2O(l) H°f = -285.8 kJ/mol
H2O2(l) H2(g)+ O2(g) H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 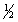 O2(g),
O2(g),
Definitions:
Geologic Time
A system of chronological measurement that relates stratigraphy to time, used by geologists, paleontologists, and other Earth scientists to describe the timing and relationships of events in Earth’s history.
Archaean
A member of the domain Archaea, a group of single-celled microorganisms that are distinct from bacteria and eukaryotes, often thriving in extreme environments.
Environmental Pressures
Various factors in the environment that can influence the survival, reproduction, and evolution of organisms, often leading to natural selection.
Heritable Changes
Alterations in a genetic material that can be passed on from one generation to another.
Q25: How many grams of nitrogen are there
Q25: Zinc dissolves in hydrochloric acid to
Q28: Complete the following table. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="Complete
Q42: Write a Lewis structure for the phosphate
Q48: In an electron microscope, electrons are accelerated
Q55: According to VSEPR theory, which one of
Q72: Calculate the density of Ar(g)at -11°C and
Q78: The Bohr model of the hydrogen atom
Q82: Calculate the amount of work done, in
Q89: A compound was discovered whose composition by