Use the Born-Haber cycle to calculate the lattice energy of KCl(s) given the following data: H(sublimation) K = 79.2 kJ/mol
I1 (K) = 418.7 kJ/mol
Bond energy (Cl-Cl) = 242.8 kJ/mol
EA (Cl) = 348 kJ/mol
H 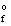 (KCl(s) ) = -435.7 kJ/mol
(KCl(s) ) = -435.7 kJ/mol
Definitions:
Overhead Assigned
The allocation of overhead costs to specific jobs or cost objects based on a predetermined rate or method.
Product X0
A specific product identifier used in inventory and production tracking, symbolizing a unique item or variant within a company's product line.
Overhead Applied
The portion of manufacturing overhead costs assigned to each unit of production, based on a predetermined rate.
Activity-Based Costing
A method of accounting that assigns costs to products or services based on the activities they require.
Q6: Gasoline (which can be considered to be
Q10: Write balanced molecular and net ionic equations
Q14: Which two electron configurations represent elements that
Q15: A possible set of quantum numbers for
Q20: A proton is roughly 1800 times more
Q34: Which one of the following molecules has
Q53: The formal charge on the sulfur atom
Q53: If the pressure of a gas sample
Q96: Which of the following properties indicates the
Q112: Write the Lewis dot symbol for the