Use the Born-Haber cycle to calculate the lattice energy of MgO (s) given the following data: H(sublimation) Mg = 130 kJ/mol
I1 (Mg) = 738.1 kJ/mol
I2 (Mg) = 1450 kJ/mol
Bond energy (O=O) = 498.7 kJ/mol
EA (O) = 141 kJ/mol
EA (O-) = -780 kJ/mol
H 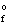 (MgO(s) ) = -601.8 kJ/mol
(MgO(s) ) = -601.8 kJ/mol
Definitions:
Mentally Disabled
A condition that limits a person's cognitive, emotional, or behavioral functioning.
Status Indians
Individuals who are legally recognized as Indians under the Canadian Indian Act, entitling them to certain rights and benefits.
Children
Individuals in the early stages of life, typically considered to be under the age of majority, which varies by jurisdiction.
Contracts
Legal agreements between two or more parties that are enforceable by law.
Q13: The van der Waals equation is a
Q18: The vapor pressure of ethanol is 400
Q28: A ground-state atom of vanadium has _
Q41: A 0.1946 g piece of magnesium metal
Q64: A piece of copper metal was added
Q78: Write the ground-state electron configuration for Mg<sup>2+</sup>.
Q94: The successive ionization energies of a certain
Q111: Which of the following species has the
Q113: For which of these species does the
Q116: Find the temperature at which ethanol boils