Use the Born-Haber cycle to calculate the standard enthalpy of formation ( H 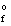 ) for LiCl(s) given the following data: H(sublimation) Li = 155.2 kJ/mol
) for LiCl(s) given the following data: H(sublimation) Li = 155.2 kJ/mol
I1 (Li) = 520 kJ/mol
Bond energy (Cl-Cl) = 242.7 kJ/mol
EA (Cl) = 349 kJ/mol
Lattice energy (LiCl(s) ) = 828 kJ/mol
Definitions:
Overhead Applied
The allocation of estimated overhead costs to individual units of production, based on a predetermined rate.
Forming Department
A section in manufacturing where raw materials are shaped or formed into parts for further production processes.
Machine-Hours
The total hours that machinery is operated during the production process.
Predetermined Overhead Rate
A rate used to allocate manufacturing overhead to individual products or jobs, based on a predetermined formula, prior to actual production.
Q3: The temperature of an ideal gas in
Q9: What is the pressure (in atmospheres)of the
Q11: Which of the following ground-state ions has
Q17: A homonuclear diatomic molecule is a molecule
Q19: How many electrons are in the 4p
Q54: Arrange the following aqueous solutions in order
Q70: The N - N - H bond
Q73: Ethanol undergoes combustion in oxygen to
Q109: The bond in which one of the
Q117: Which of the elements listed below has