Calculate the Energy Change for the Reaction K(g)+ I(g) K+(g)+ I - (G)
Given the Following Ionization Energy (IE)and
Calculate the energy change for the reaction K(g) + I(g) K+(g) + I - (g)
Given the following ionization energy (IE) and electron affinity (EA) values. 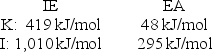
Definitions:
Z Problem-Solving Model
A structured approach to address and solve problems by systematically considering various aspects and solutions, ensuring thorough analysis and decision-making.
Gathering Information
The process of collecting data or insights to inform decisions or understand situations.
Sensing
Gathering information through the five senses and focusing on what actually exists.
Escalation of Commitment
Describes a pattern of behavior in which individuals continue to invest time, money, or effort into a decision or action despite evidence of its failure, often due to a desire to justify prior commitments.
Q11: A 0.3423 g sample of pentane, C<sub>5</sub>H<sub>12</sub>,
Q12: Which of the following substance is/are planar?
Q20: Which of the following Lewis structures is
Q41: Assuming the octet rule is obeyed, how
Q60: Which of the following is the electron
Q75: Calculate the enthalpy of reaction for
Q77: A beaker contains 115 g of ethanol
Q90: The triple point of iodine is at
Q91: If 2Mg(s)+ O<sub>2</sub>(g) <span class="ql-formula" data-value="\rarr"><span
Q140: What mass of water would need