Use the Born-Haber cycle to calculate the lattice energy of KCl(s) given the following data: H(sublimation) K = 79.2 kJ/mol
I1 (K) = 418.7 kJ/mol
Bond energy (Cl-Cl) = 242.8 kJ/mol
EA (Cl) = 348 kJ/mol
H 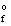 (KCl(s) ) = -435.7 kJ/mol
(KCl(s) ) = -435.7 kJ/mol
Definitions:
Testosterone Levels
The concentration of the hormone testosterone in the bloodstream, which plays a key role in male development and health.
Symptoms
Signs or indications of a condition or disease, observed by a healthcare professional or reported by the patient.
Increased Libido
A heightened drive or desire for sexual activity.
Menopause
Marks the end of a woman's menstrual cycles, diagnosed after 12 consecutive months without a menstrual period, signifying the end of reproductive years.
Q5: Give the number of lone pairs around
Q6: Given that the heat of vaporization of
Q6: Which one of the following is most
Q25: For the overall chemical reaction shown below,
Q40: According to VSEPR theory, which of the
Q45: A convenient way to produce very
Q47: The solubility of CO<sub>2</sub> gas in water<br>A)increases
Q67: How many grams of propanol (C<sub>3</sub>H<sub>7</sub>OH, 60.10
Q92: For the hypothetical reaction A +
Q117: What is the hybridization on the central