Use the Born-Haber cycle to calculate the lattice energy of MgO (s) given the following data: H(sublimation) Mg = 130 kJ/mol
I1 (Mg) = 738.1 kJ/mol
I2 (Mg) = 1450 kJ/mol
Bond energy (O=O) = 498.7 kJ/mol
EA (O) = 141 kJ/mol
EA (O-) = -780 kJ/mol
H 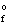 (MgO(s) ) = -601.8 kJ/mol
(MgO(s) ) = -601.8 kJ/mol
Definitions:
Applied Overhead
The portion of overhead costs allocated to specific jobs or production activities based on a predetermined formula or rate.
Department 1
A generic term that may refer to the first division or unit within an organization or company, often associated with a specific function or set of responsibilities.
Commissions Paid
Payments made to employees or agents based on their sales volume or performance.
Cost Trends
Patterns or movements in cost over a period of time, indicating how expenses are changing and affecting profitability.
Q11: The electron dot formula for O<sub>2</sub> shows<br>A)a
Q30: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="For the
Q35: Which one of the following molecules has
Q44: Use the Born-Haber cycle to calculate
Q65: Palladium crystallizes in a face-centered cubic unit
Q71: Methanol (CH<sub>3</sub>OH)burns according to the equation
Q90: A certain first-order reaction A <font face="symbol"></font>
Q92: A sample of hydrogen gas was collected
Q93: Naphthalene combustion can be used to calibrate
Q95: Consider a 0.90 M Al(NO<sub>3</sub>)<sub>3</sub> solution. This