Use the Born-Haber cycle to calculate the standard enthalpy of formation ( H 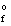 ) for LiCl(s) given the following data: H(sublimation) Li = 155.2 kJ/mol
) for LiCl(s) given the following data: H(sublimation) Li = 155.2 kJ/mol
I1 (Li) = 520 kJ/mol
Bond energy (Cl-Cl) = 242.7 kJ/mol
EA (Cl) = 349 kJ/mol
Lattice energy (LiCl(s) ) = 828 kJ/mol
Definitions:
Organ of Corti
The sensory organ located within the cochlea of the inner ear, responsible for the transduction of sound vibrations into electrical signals.
Pituitary Gland
A small, pea-sized gland located at the base of the brain, crucial in controlling growth, metabolism, and reproductive function.
Endocrine Glands
Glands that secrete hormones directly into the bloodstream to regulate various body functions.
Variety of Hormones
Refers to the diverse group of chemical messengers produced by endocrine glands that regulate bodily functions, including growth, metabolism, and reproduction.
Q25: The polarity of covalent bonds increases as
Q45: Which one of the following elements forms
Q63: Indicate all the types of intermolecular forces
Q65: Liquid nitrogen has a density of 0.807
Q83: Calculate the frequency of visible light having
Q83: Samples of the following volatile liquids are
Q94: Which of the following substances would have
Q94: What is the energy in joules of
Q95: Consider a 0.90 M Al(NO<sub>3</sub>)<sub>3</sub> solution. This
Q109: An endothermic reaction causes the surroundings to<br>A)warm