Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 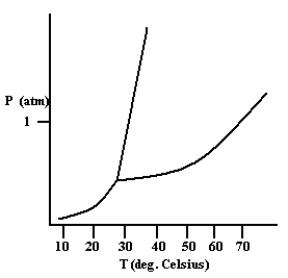
Definitions:
Compounded Annually
Interest calculation method where the interest is added to the principal sum at the end of each year, so that the balance also earns interest in the following year.
Yearly Contributions
Refers to the payments or investments made annually into a plan or fund, often for retirement or savings purposes.
Interest Rate
The cost of borrowing money or the return on investment, usually expressed as a percentage of the principal amount per period of time.
Borrower's Payment
The amount of money paid by a borrower, typically on a regular schedule, to repay or service a debt.
Q9: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH)will have a greater
Q37: Which one of the following is not
Q43: Dissolving a solute such as KOH in
Q57: The alkali metal elements are found in
Q58: What type of chemical bond holds the
Q63: Pure benzene, C<sub>6</sub>H<sub>6</sub>, freezes at 5.5° and
Q74: Calculate the freezing point of a solution
Q85: For the reaction 2NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="For
Q91: Which of the following correctly lists species
Q114: Lanthanide (or rare earth elements)have atoms or