The isomerization of cyclopropane to propene follows first-order kinetics. 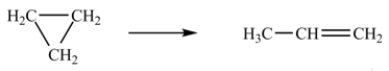 At 700 K, the rate constant for this reaction is 6.2 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
At 700 K, the rate constant for this reaction is 6.2 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
Definitions:
Professional Practice
The act of engaging in an occupation as a professional, adhering to its standards, ethics, and responsibilities.
Philosophical Claims
Assertions that pertain to the study of the fundamental nature of knowledge, reality, and existence, often as part of a wider ideology or theory.
Formal Nursing Knowledge
The body of theory, principles, and facts that forms the scientific basis for nursing practices and procedures.
Parse
To analyze a string or text into its component parts, often done in programming to understand or process input data.
Q14: For the reaction X + Y <font
Q18: The molar solubility of magnesium carbonate is
Q34: Hydrogen iodide decomposes according to the equation:<br>2HI(g)
Q47: For the chemical reaction A <font face="symbol"></font>
Q63: Indicate all the types of intermolecular forces
Q83: In which of the forms listed below
Q94: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,
Q97: A saturated sodium carbonate solution at 100°C
Q101: Which of the following concentration units will
Q114: Which one of the following substances crystallizes