At 700 K, the rate constant for the following reaction is 6.2 10-4 min-1. 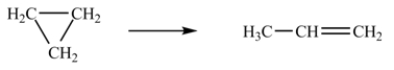 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
Definitions:
Empowerment
The process of enabling individuals or groups to gain control, authority, and influence over their own lives or work.
Work Style
The manner or approach an individual takes towards completing tasks or organizing work, often reflecting personal preferences and strengths.
Great Leader
An individual who possesses exceptional leadership qualities, inspiring others towards achieving a common goal effectively.
Empathy
The ability to understand and share the feelings of another individual.
Q27: Which of the following correctly lists species
Q28: Which of the following covalent bonds is
Q33: Nitrous oxide (N<sub>2</sub>O)decomposes at 600°C according to
Q42: Which of the responses includes all of
Q51: Consider the hypothetical element Y with electron-dot
Q57: Will a 0.1 M solution of Na<sub>2</sub>HPO<sub>4</sub>(aq)be
Q69: Which response gives the products of hydrolysis
Q90: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q102: The total number of bonding electrons in
Q143: Arrange the acids HOCl, HClO<sub>3</sub>, and HClO<sub>2</sub>