For the chemical reaction system described by the diagram below, which statement is true? 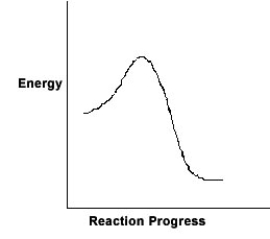
Definitions:
Addition Rule
A method used in probability to determine the total probability of the occurrence of at least one of several events.
Deck of Cards
A standard set of 52 playing cards, used in various games, consisting of four suits: hearts, diamonds, clubs, and spades.
Addition Rule
In probability, a rule that calculates the likelihood of any of two or more mutually exclusive events occurring.
Automobile Accident
An unexpected event involving a vehicle that usually results in damage and/or injury.
Q11: In order for a gas to be
Q15: Write a Lewis structure for the chlorate
Q18: Write a Lewis structure for SO<sub>3</sub> that
Q62: When acetaldehyde at a pressure of 364
Q78: Indicate all the types of intermolecular forces
Q82: The pH of a certain solution is
Q109: Boron nitride, BN<sub>3</sub>, melts at approximately at
Q111: Indicate all the types of intermolecular forces
Q113: Of the two acids HBr and H<sub>2</sub>Se,
Q115: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many