For the reaction SO2(g) + NO2(g) 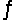 SO3(g) + NO(g) , the equilibrium constant is 18.0 at 1,200ºC. If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20.L container, what concentration of SO3 will be present at equilibrium?
SO3(g) + NO(g) , the equilibrium constant is 18.0 at 1,200ºC. If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20.L container, what concentration of SO3 will be present at equilibrium?
Definitions:
Deer
A wild or domestically raised animal with antlers found in various regions, known for its agility and grace.
Plastic
A synthetic material made from a wide range of organic polymers, such as polyethylene and nylon, that can be molded into shape while soft and then set into a rigid or slightly elastic form.
Wood
A fibrous material made from the trunks and branches of trees, used for construction, paper production, and as a fuel source.
Bagel Press
A kitchen tool or appliance designed to shape bagel dough uniformly before it is boiled and baked.
Q7: What volume of 0.200 M potassium hydroxide
Q39: Which one of these salts will form
Q44: Calculate the pH of a buffer solution
Q45: Phosgene, COCl<sub>2</sub>, a poisonous gas, decomposes according
Q53: Which of the following characteristics indicates the
Q66: Give the number of lone pairs around
Q67: Potassium bromide, KBr, crystallizes like NaCl in
Q89: The graphs below all refer to the
Q104: What is the freezing point of a
Q117: What is the hybridization on the central