On analysis, an equilibrium mixture for the reaction 2H2S(g) 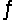 2H2(g) + S2(g) was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel. Calculate the equilibrium constant, Kc, for this reaction.
2H2(g) + S2(g) was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel. Calculate the equilibrium constant, Kc, for this reaction.
Definitions:
Liability
A financial obligation or debt that a company owes, which must be settled over time through the transfer of economic benefits including money, goods, or services.
Withdrawing Partner
An individual partner who exits a partnership, thereby receiving their share of the partnership's assets after settling any liabilities.
Accounts Receivable
Money owed to a business by its clients or customers for goods or services delivered but not yet paid for, recorded as an asset on the balance sheet.
Allowance for Doubtful Accounts
A contra-asset account used to estimate the amount of receivables that may not be collectible.
Q2: Ozone is formed in the atmosphere by
Q27: The free energy of formation of nitric
Q43: Acid strength decreases in the series HI
Q56: The oxidation of iodide ions by arsenic
Q60: Consider the two gaseous equilibria: <br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q64: The osmotic pressure of a 0.010 M
Q73: Ozone (O<sub>3</sub>)in the atmosphere can reaction
Q87: What is the pH of an aqueous
Q110: A nuclear stress test utilizes a gamma-emitting
Q142: Which one of these statements about strong