Equilibrium is established for the reaction 2X(s) + Y(g) 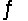 2Z(g) at 500K, Kc = 100.Determine the concentration of Z in equilibrium with 0.2 mol X and 0.50 M Y at 500K.
2Z(g) at 500K, Kc = 100.Determine the concentration of Z in equilibrium with 0.2 mol X and 0.50 M Y at 500K.
Definitions:
Q6: The compound CFCl<sub>3</sub> is used as a/an<br>A)enzyme<br>B)anesthetic<br>C)gaseous
Q26: What mass of solid CaO is
Q39: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q47: Which one of the following is a
Q60: Consider the two gaseous equilibria: <br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q81: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q100: Of the given pair of compounds, which
Q100: According to VSEPR theory, which one of
Q102: When the concentrations of reactant molecules are
Q107: Which one of the following reactions