At 400ºC, Kc = 64 for the equilibrium H2(g) + I2(g) 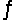 2HI(g) . If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.
2HI(g) . If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.
Definitions:
Retirement Facilities
Specialized housing designed to meet the needs of the elderly, often providing medical care, social activities, and support services.
Quad-Base Cane
A walking aid featuring a four-footed base providing increased stability for users.
Nonskid Shoes
Footwear designed to increase traction and reduce the likelihood of slipping.
Q17: Calculate the concentration of malonate ion (C<sub>3</sub>H<sub>2</sub>O<sub>4</sub><sup>2-</sup>)in
Q23: The solubility product for chromium(III)fluoride is K<sub>sp</sub>
Q31: A 0.10 M NH<sub>3</sub> solution is 1.3%
Q35: How many moles of NaF must be
Q37: The CO<sub>2</sub> content of Earth's atmosphere is
Q96: What is the molar mass of toluene
Q103: The equilibrium constant for the reaction
Q106: What is the pH at the equivalence
Q119: If a triatomic molecule is linear, then
Q135: Each of the following substances is a