For the following reaction at equilibrium, which one of the changes below would cause the equilibrium to shift to the left? 2NOBr(g) 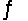
2NO(g) + Br2(g) , Hºrxn = 30 kJ/mol
Definitions:
Pleasure Principle
A concept in psychoanalytic theory suggesting that people seek pleasure and avoid pain, guiding the id's desire to satisfy basic urges, needs, and desires.
Reality Principle
A psychoanalytic concept where the ego negotiates between the desires of the id and the super-ego, often requiring a compromise to deal with the demands of reality.
Personality
The combination of characteristics or qualities that form an individual's distinctive character.
Enduring
Enduring refers to lasting over a long period of time, demonstrating persistence or resilience in the face of challenges.
Q10: Identify the dominant (strongest)type of intermolecular force
Q36: Explain the following, on the basis of
Q55: Identify the conjugate acid of SO<sub>4</sub><sup>2-</sup> in
Q63: Indicate all the types of intermolecular forces
Q79: Will a precipitate form (yes or no)when
Q92: Which of the following would be expected
Q99: Which one of the following molecules has
Q106: What is the pH at the equivalence
Q109: Boron nitride, BN<sub>3</sub>, melts at approximately at
Q136: Which of these lists of molecules is