For the following reaction at equilibrium in a reaction vessel, which one of these changes would cause the Br2 concentration to decrease? 2NOBr(g) 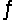
2NO(g) + Br2(g) , Hºrxn= 30 kJ/mol
Definitions:
Concrete Reasoning
Thinking based on the physical, tangible world and immediate experiences rather than abstraction or hypothetical scenarios.
Formal Reasoning
The process of logical thinking and problem-solving that follows established rules and procedures, typically associated with mature cognitive development.
Analytic Thought
A cognitive process that involves breaking down complex information or problems into smaller parts or basic principles for better understanding and problem-solving.
Deductive Reasoning
A logical process where a conclusion follows necessarily from the stated premises, moving from general premises to a specific conclusion.
Q22: When 20.0 grams of an unknown compound
Q26: Will a precipitate (ppt)form when 300.mL of
Q27: Calculate the concentration of chloride ions in
Q55: The vapor pressure of a liquid in
Q70: Calculate the pOH of a solution containing
Q71: Which of these acids is stronger, H<sub>3</sub>AsO<sub>3</sub>
Q80: Calculate the percent ionization of cyanic acid,
Q87: Which of the following atoms does not
Q96: What is the molar mass of toluene
Q152: What mass of sodium formate (HCOONa)must be