For the following reaction at equilibrium in a reaction vessel, which one of these changes would cause the Br2 concentration to increase? 2NOBr(g) 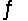
2NO(g) + Br2(g) , Hºrxn= 30 kJ/mol
Definitions:
Adaptive Personalities
Traits in individuals that enhance their ability to cope with changes and challenges in their environment.
Self-awareness
The conscious knowledge of one's own character, feelings, motives, and desires.
Temporal Orientation
An individual's or culture's attitudes and perceptions regarding time, emphasizing past, present, or future.
Industrialized Countries
Nations that have undergone substantial industrial development, characterized by significant manufacturing, advanced technological infrastructure, and higher standards of living.
Q25: Will a precipitate (ppt)form when 20.0 mL
Q32: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q38: Without reference to a table, arrange
Q47: The solubility of CO<sub>2</sub> gas in water<br>A)increases
Q50: Calculate the percent ionization of formic acid
Q65: Palladium crystallizes in a face-centered cubic unit
Q72: The solubility product for calcium phosphate is
Q83: In the reaction Ag<sup>+</sup>(aq)+ Cl<sup>-</sup>(aq) <span
Q95: The pH of a solution that is
Q117: Which one of the following salts will