For the reaction at equilibrium 2SO3 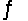 2SO2 + O2 ( Hºrxn= 198 kJ/mol) , if we increase the reaction temperature, the equilibrium will
2SO2 + O2 ( Hºrxn= 198 kJ/mol) , if we increase the reaction temperature, the equilibrium will
Definitions:
Liberal Intellectuals
Thinkers and scholars who advocate for progressivism, freedom, equality, and the expansion of civil rights, often engaging in political, social, and cultural analysis.
Civil Liberties
Fundamental individual rights protected by law against unwarranted governmental or other interference, including freedom of speech, right to privacy, and the right to a fair trial.
Progressive Era
A period of widespread social activism and political reform in the United States, from the 1890s to the 1920s, targeting issues like corruption, inequalities, and economic reforms.
New Deal Reforms
A series of programs, public work projects, financial reforms, and regulations enacted by President Franklin D. Roosevelt in the United States during the 1930s aimed at restoring prosperity and helping Americans during the Great Depression.
Q6: Given that the heat of vaporization of
Q18: Complete and balance the following redox
Q21: What is the freezing point of an
Q31: The normal freezing point of ammonia
Q36: What is the effective pH range for
Q42: Hydrogen peroxide decomposes to water and oxygen
Q81: The freezing point of a liquid does
Q97: A saturated sodium carbonate solution at 100°C
Q100: When 24.0 g of glucose (a nonelectrolyte)are
Q119: Identify the dominant (strongest)type of intermolecular force