Consider this gas phase equilibrium system: PCl5(g) 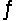
PCl3(g) + Cl2(g) Hºrxn = +87.8 kJ/mol.
Which of these statements is false?
Definitions:
Seat Occupancy
The ratio or percentage of occupied seats in a venue or vehicle to the total available.
Operating Performance
An evaluation of a company's efficiency in managing its core business activities, often measured by profitability and productivity metrics.
Fixed Manufacturing Expenses
Costs that do not vary with the level of production or sales, such as rent, salaries of permanent staff, and equipment depreciation.
Fixed Selling
Refers to selling expenses that remain constant regardless of the level of sales or production activity.
Q13: Which of the following cannot be plausibly
Q37: Nitrogen pentoxide decomposes by a first-order process
Q40: Excluding water vapor, list the four most
Q41: Explain the following, on the basis of
Q48: Which one of the following units would
Q55: Identify the conjugate acid of SO<sub>4</sub><sup>2-</sup> in
Q68: Identify the conjugate acid-base pairs in the
Q96: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)+ 2296
Q97: Which response includes all the following
Q110: Which of these species has the highest