Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) 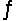
2SO3(g)
Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be
Definitions:
Probability
Quantifying the potential that an event occurs, with numbers from 0 to 1 representing the range.
Occurrence
The fact or frequency of something happening.
Affected
Being influenced or changed by an external factor.
Probability
A measure of the likelihood or chance that a particular event will occur, expressed as a number between 0 and 1.
Q13: In which one of the following solutions
Q38: Which of the following is a catalyst
Q53: Calculate K<sub>c</sub> for the reaction 2HI(g)<sub> </sub>
Q62: Acetic acid has a heat of fusion
Q74: Using the thermodynamic data provided below, determine
Q78: Indicate all the types of intermolecular forces
Q81: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q98: Appropriate units for a first-order rate constant
Q114: Which one of these salts will form
Q123: The number of nearest neighbors (atoms that