When the substances in the equation below are at equilibrium, at pressure P and temperature T, the equilibrium can be shifted to favor the products by CuO(s) + H2(g) 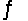
H2O(g) + Cu(s) Hºrxn = -2.0 kJ/mol
Definitions:
1934 Act
Refers to the Securities Exchange Act of 1934, which governs the trading of securities in the U.S., including the establishment of the SEC.
Registration Under
The process of officially recording or enlisting something or someone under certain conditions or regulations, often related to securities laws.
1933 Act
A U.S. federal law, officially known as the Securities Act of 1933, enacted to ensure more transparency in financial statements to protect investors from fraud.
Working Capital
The difference between a company's current assets and its current liabilities, indicative of its operational liquidity.
Q3: Hydrogen iodide decomposes according to the equation
Q6: The compound CFCl<sub>3</sub> is used as a/an<br>A)enzyme<br>B)anesthetic<br>C)gaseous
Q17: Which one of the following would slow
Q19: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q42: The density of a 20.3 M CH<sub>3</sub>OH
Q73: Write a net ionic equation for the
Q96: Which should have the longer bond, B<sub>2</sub>
Q98: Automobile radiators usually carry a sticker that
Q121: Which of the following is not an
Q136: Which one of the following substances should