A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively.These ions react according to the following chemical equation 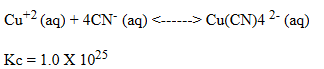
What will be the concentration of Cu2+(aq)at equilibrium?
Definitions:
Equation of Exchange
An economic equation (MV = PQ) that relates the money supply (M) and its velocity (V) to the price level (P) and quantity of goods sold (Q).
MV = PQ
An equation of exchange in economics that states money supply times the velocity of money equals the price level times the output (quantity of goods and services produced).
Big Government
A term referring to a government or state perceived as overly involved in the various aspects of societal, economic, and personal lives of its citizens.
Keynesians
Supporters of the economic theories established by John Maynard Keynes, focusing on total spending in the economy and its effects on output and inflation.
Q3: What phase exists at the point labeled
Q12: The reaction 2SO<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="The reaction
Q37: The pH of a sample of river
Q39: A negative sign for <span
Q61: A certain reaction A <font face="symbol"></font> products
Q62: When acetaldehyde at a pressure of 364
Q85: All indicators are weak acids that are
Q90: Maple syrup is mostly a solution of
Q95: Which of the following is not true
Q120: Indicate the type of hybrid orbitals used