Consider the chemical reaction 2NH3(g) 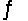
N2(g)+ 3H2(g).The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present.Estimate the equilibrium concentration of H2(g).
Definitions:
Legal Principles
Foundational rules or concepts that guide the legal system and the administration of justice.
Accreditation
The formal recognition provided to an institution or program for meeting predefined standards.
Operative Procedure
Any medical operation carried out to diagnose or treat illness or injury, involving direct manipulation or surgery on the body.
Consent Form
A document that is signed by a patient or participant, giving official permission for a medical procedure, treatment, or participation in a study.
Q2: Ozone is formed in the atmosphere by
Q20: The gas phase reaction of nitrogen dioxide
Q38: Hydrogen iodide decomposes according to the equation
Q55: What is the molality of a solution
Q61: Calculate the amount of enthalpy required
Q77: A 8.0 M solution of formic acid
Q96: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)+ 2296
Q118: For which of the following species are
Q139: Consider the weak bases below and their
Q162: For maleic acid, HOOCCH=CHCOOH, K<sub>a1</sub> = 1.42