Consider the following equilibrium,
4NH3(g)+ 3O2(g) 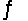
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant after ammonia was added to the system.
Definitions:
Pudendal Block
A regional anesthesia administered in the pudendal nerve area to relieve pain during childbirth.
Epidural Block
A medical procedure that involves injecting anesthetic near the spinal cord to block pain, commonly used during childbirth.
Insistent Cry
A persistent and forceful crying in infants, which may indicate distress, discomfort, or the need for attention from caregivers.
Anoxia
A condition characterized by an absence of oxygen supply to an organ or a tissue, potentially causing damage or dysfunction.
Q5: Calculate the concentration of chromate ion (CrO<sub>4</sub><sup>2-</sup>)in
Q12: The reaction 2SO<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="The reaction
Q13: Aluminum forms a layer of aluminum
Q29: Which one of the following substances crystallizes
Q43: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q54: Solid sodium iodide is slowly added to
Q59: In which of the following would the
Q67: How many grams of propanol (C<sub>3</sub>H<sub>7</sub>OH, 60.10
Q85: Which response gives the products of hydrolysis
Q98: Methane has a heat of fusion of