Kc for the reaction CO2(g)+ H2(g) 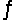
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of water in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Offerings
Refers to the products or services provided by a business to its customers.
Earn Income
The process of receiving money as payment for work performed, or from investments.
B2C Marketing
Business-to-Consumer marketing, which focuses on selling products and services directly to individual customers.
Place Element
One of the four Ps in the marketing mix, referring to the strategies and processes used to make the product or service available to the target customer.
Q14: Find the temperature at which K<sub>p</sub>
Q18: The vapor pressure of ethanol is 400
Q36: For the reaction PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q36: What is the effective pH range for
Q43: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q51: Calculate E°<sub>cell</sub> for a silver-aluminum cell
Q70: A 100.mL sample of water is taken
Q88: Which one of the following elements would
Q102: The molar solubility of tin(II)iodide is 1.28
Q123: An aqueous solution of KCl would be<br>A)neutral<br>B)basic<br>C)acidic