A 0.10 M NH3 solution is 1.3% ionized. Calculate the H+ ion concentration. NH3 + H2O 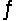 NH4+ + OH-
NH4+ + OH-
Definitions:
Criteria
Standards or principles by which something is judged or decided.
Conceptions
Ideas or mental representations of how something works or is structured.
Indirect Leadership
A style of leadership where the leader influences others through indirect means, such as setting a vision, creating an environment conducive to growth, and inspiring via example, rather than direct command and control.
Emailing
A method of exchanging digital messages between people using electronic devices.
Q19: Calculate K<sub>p</sub> at 298 K for
Q47: If the pH of pure water is
Q56: Which of these metals will reduce water
Q60: The measured voltage of a cell
Q70: An environmental chemist obtained a 200.mL sample
Q79: Will a precipitate form (yes or no)when
Q85: An electrochemical cell based on the
Q86: What is the net ionic equation
Q92: In the gas phase, formic acid
Q101: What phase exists at the point labeled