Calculate the equilibrium constant Kc for the net reaction shown below.
AgI(s)+ 2NH3(aq) 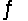 Ag(NH3)2+(aq)+ I-(aq)
Ag(NH3)2+(aq)+ I-(aq)
For AgI, Ksp = 8.3 * 10-17; for Ag(NH3)2+, Kf = 1.5 * 107.
Definitions:
Strange Situation
A research method used in developmental psychology to assess attachment relationships between caregivers and children.
Attachment Differences
Variations in the way individuals form and maintain emotional bonds with others, frequently explored in the context of parent-child relationships.
Formal Operational
The last stage in Piaget's theory of cognitive development, characterized by the ability to think abstractly, logically, and systematically.
Schema
An intellectual structure or idea that aids in categorizing and comprehending data.
Q6: An experimental drug, D, is known to
Q12: Which one of the following can function
Q13: Calcium metal is produced by electrolysis of<br>A)CaSO<sub>4</sub><br>B)CaF<sub>2</sub><br>C)CaCO<sub>3</sub><br>D)Ca(OH)<sub>2</sub><br>E)CaCl<sub>2</sub>
Q16: Write the chemical formula of pyrite.
Q29: Given the following data, estimate the
Q37: When phosphate rock, Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>(s), is converted to
Q45: The activation energy for the reaction O
Q67: Consider the reaction N<sub>2</sub>(g)+ O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q75: A saturated sodium carbonate solution at 0°C
Q163: If the pH of stomach acid is