Aluminum forms a layer of aluminum oxide when exposed to air which protects the bulk metal from further corrosion. 4Al(s) + 3O2(g) 2Al2O3(s)
Using the thermodynamic data provided below, calculate S° for this reaction. 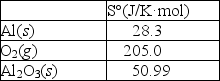
Definitions:
Pull Method
A manufacturing and inventory strategy where production is based on customer demand rather than forecasting.
Work-In-Process Inventory
Goods that are in various stages of the production process but are not yet completed.
Non-Value-Added Cost
Expenses that do not contribute to the value or functionality of a product or service, often targeted for reduction or elimination.
Market Research
The process of gathering, analyzing, and interpreting information about a market, including the product or service's potential customers and competitors.
Q9: The isotope with the greatest nuclear binding
Q23: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q25: The <sup>14</sup>C activity of some ancient Peruvian
Q29: How many coulombs (C)of electrical charge must
Q34: Which one of these species exists as
Q42: In comparing three solutions with pH's of
Q58: Which of these square planar complex ions
Q61: Write a net ionic equation for the
Q71: Solid sodium iodide is slowly added to
Q91: Bromothymol blue is a common acid-base indicator.