Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium nitrate is dissolved in water? 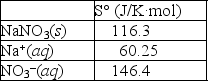 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Definitions:
Par Value
The nominal or face value of a stock or bond as stated by the issuer, which may differ from its market value.
Coupon Rate
The yearly interest rate provided by a bond, shown as a percentage of the bond's nominal value.
Yield To Maturity
Yield to maturity represents the total return an investor would receive by holding a bond until it matures, including all interest payments and the repayment of principal.
Realized Compound Yield
The reflective measurement of the total returns on an investment, factoring in compounding interest or returns over a specified time frame.
Q3: A naturally occurring substance with a range
Q11: When comparing acid strength of binary acids
Q19: Which one of these hydrocarbons does not
Q20: What molar ratio of benzoate ion to
Q36: Calculate the binding energy per nucleon of
Q61: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="Balance the
Q64: How long will it take to
Q73: Complete and balance the following redox
Q105: Find the temperature at which K<sub>p</sub>
Q111: What is the OH<sup>-</sup> ion concentration in