Calculate S° at 25°C for the reduction of PbO(s) , 2PbO(s) + C(s) 2Pb(s) + CO2(g) given these absolute entropies: 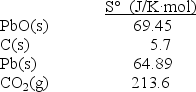
Definitions:
Values
Core beliefs or standards that guide individuals' actions and judgments, influencing behavior and attitudes.
Learning To Say No
The process or skill of setting personal or professional boundaries by declining requests or demands that exceed one’s capacity or value system.
Other Person's Perspective
The consideration and understanding of someone else's viewpoint or position in a situation or discussion.
Stress Prevention
Strategies and measures implemented to reduce or eliminate stressors in the environment to improve wellbeing and productivity.
Q8: For the reaction represented below, the experimental
Q8: Solid sodium iodide is slowly added to
Q11: Which one of these elements is not
Q15: The electron configuration of a Co<sup>3+ </sup>ion
Q20: An increase in the amount of particulate
Q35: Write an equation for a laboratory preparation
Q43: Choose the substance with the higher entropy
Q73: Write a net ionic equation for the
Q87: Aluminum does not corrode as does iron,
Q113: Consider an electrochemical cell involving the