Calculate the equilibrium constant for the decomposition of water 2H2O(l) 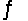 2H2(g) + O2(g)
2H2(g) + O2(g)
At 25°C, given that G°f (H2O(l) ) = -237.2 kJ/mol.
Definitions:
Purchase price
The price at which an item or property is bought.
Conditional sale contract
A sales agreement that transfers ownership of an item to the buyer only after specific conditions are met, usually full payment of the purchase price.
Compounded monthly
The process of calculating interest on both the initial principal and the accumulated interest from previous periods, done on a monthly basis.
Payment-free period
A specified duration during which a borrower is not required to make any payments on a loan.
Q7: The flotation process used in metallurgy involves<br>A)the
Q10: The Ostwald process is the main method
Q12: The mineral cryolite, Na<sub>3</sub>AlF<sub>6</sub>, is used in
Q14: Which one of the following indoor air
Q25: Consider the following standard reduction potentials in
Q27: A possible reaction leading to removal
Q62: Predict which nucleus is less stable, <img
Q63: Using the thermodynamic data provided below, calculate
Q88: What is the pH at the equivalence
Q94: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,