Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium nitrate is dissolved in water? 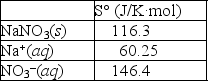 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Definitions:
Patients' Understanding
The level at which individuals comprehend their health conditions, treatment plans, and healthcare instructions.
Noncompliance
The act of not following prescribed rules, regulations, or advice.
Physicians
Medical professionals who diagnose and treat illnesses, manage patient care, and offer guidance on maintaining health.
Serious Illnesses
Conditions that are potentially life-threatening or that can cause significant impairment to quality of life.
Q7: The correct formula for the dibromobis(oxalato)cobaltate(III)ion is
Q22: A molecule or atom that accepts an
Q34: Hydrogen iodide decomposes according to the equation:<br>2HI(g)
Q55: The equilibrium constant for the chemical equation<br>2NO(g)+
Q67: Hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)decomposes according to the
Q70: An environmental chemist obtained a 200.mL sample
Q72: Consider the reaction Fe + Sn<sup>2+</sup>(1
Q91: Bromothymol blue is a common acid-base indicator.
Q93: At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q102: What role does cadmium metal (Cd)play in