Essay
Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium sulfate is dissolved in water? 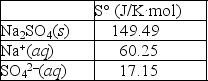 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Definitions:
Related Questions
Q12: A standard hydrogen electrode is immersed in
Q13: Which of the following cannot be plausibly
Q25: The normal process occurring on Earth that
Q29: How many coulombs (C)of electrical charge must
Q38: Hydrogen iodide decomposes according to the equation
Q54: Predict the sign of <span
Q58: Metallic sodium is obtained commercially from molten
Q59: Which of these species will act as
Q60: What is the coordination number of cobalt
Q68: Consider an electrochemical cell based on