For the reaction SbCl5(g) 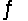 SbCl3(g)+ Cl2(g),
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Definitions:
Medical Care
Professional health services provided by doctors, nurses, or other healthcare practitioners aimed at diagnosing, treating, and preventing illnesses and injuries.
Severe Symptoms
Intense or extreme signs of illness or disorder that significantly impact an individual's health or functioning.
Stressful Medical Procedure
Any medical or surgical intervention that induces significant stress or anxiety in patients.
Internal Body Status
A term referring to the ongoing physiological conditions and functioning of the body's internal organs and systems.
Q2: Describe how to make a sodium formate
Q13: Complete and balance the following redox
Q19: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q26: How would you expect the molecule 1,10-phenanthroline
Q45: Complete and balance the following redox
Q64: Predict the other product of the
Q67: Which response has both answers correct? Will
Q90: The mass of a nucleus is always
Q95: Which one of these net ionic
Q99: How many grams of chromium would