Consider the reaction CO(g)+ 2H2(g) 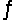 CH3OH(l)at 25°C.
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate G° at 25°C.
Definitions:
Fallacy
An incorrect reasoning process that results in the invalidity of an argument.
Raccoons
Medium-sized mammals known for their distinctive facial mask and ringed tail, often found in North America.
Dangerous Animals
Animals that pose a threat to human safety due to their behavior, physical capabilities, or potential for transmitting diseases.
Pet Raccoon
Refers to the uncommon practice of keeping a raccoon, a typically wild animal known for its dexterity and intelligence, as a domestic pet.
Q10: Name the complex ion [CuCl<sub>3</sub>Br(NH<sub>3</sub>)<sub>2</sub>]<sup>2-</sup>.
Q11: The expected product from the addition of
Q13: Calcium metal is produced by electrolysis of<br>A)CaSO<sub>4</sub><br>B)CaF<sub>2</sub><br>C)CaCO<sub>3</sub><br>D)Ca(OH)<sub>2</sub><br>E)CaCl<sub>2</sub>
Q17: The total number of electrons in the
Q31: The concentration of Mg<sup>2+</sup> in seawater is
Q42: What is the decay rate of
Q85: All indicators are weak acids that are
Q86: When an aqueous solution of AgNO<sub>3</sub> is
Q89: The data below refer to the following
Q96: The electrochemical cell that utilizes the