Consider the following standard reduction potentials in acid solution: 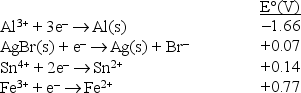 The strongest oxidizing agent among those shown above is
The strongest oxidizing agent among those shown above is
Definitions:
Standardizing Work
The process of developing and implementing uniform procedures for tasks and operations to improve efficiency, consistency, and quality.
Highly Specialized
Pertaining to or involving specific expertise or focus on a narrow area of knowledge or activity, often requiring advanced skills or training.
Unpleasant Outcome
A result of actions or decisions that is negative or less favorable than anticipated, causing dissatisfaction or adverse effects.
Verbal Reprimand
A disciplinary action involving a spoken warning or rebuke to an employee for unsatisfactory performance or behavior.
Q17: Which one of the following would slow
Q17: Aluminum is an active metal, but does
Q30: Consider the reaction Fe + Sn<sup>2+</sup>(1
Q36: Which of these species is not an
Q40: Ethylenediaminetetraacetic acid (EDTA)is<br>A)not useful as a chelating
Q68: Determine the equilibrium constant (K<sub>p</sub>)at 25°C
Q78: The half-life of <sup>90</sup>Sr is 29 years.
Q90: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q125: Which element is associated with the term
Q135: Complete and balance the following redox