Determine the equilibrium constant, Keq, at 25°C for the reaction 2Br- (aq) + I2(s) 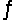
Br2(l) + 2I- (aq)
Definitions:
Negotiation Process
A methodical approach to reaching a mutually acceptable agreement between two or more parties, involving communication and compromise.
Formal Agreement
A legally binding contract or arrangement between two or more parties that is documented and enforceable by law.
High Trust
Refers to the confidence in the reliability, integrity, and abilities of a person, organization, or system.
Take Risks
The action of engaging in activities that carry a potential for failure, but also for significant reward or positive outcome.
Q10: Which choice lists both the sugar and
Q15: The electron configuration of a Co<sup>3+ </sup>ion
Q15: Predict the normal boiling point of triethylborane
Q18: Write the formula for the alcohol and
Q48: Write a balanced chemical equation illustrating chemical
Q59: Which response includes all of the
Q75: A complex with the composition [MA<sub>2</sub>B<sub>2</sub>]X<sub>2</sub> is
Q76: Will a precipitate (ppt)form when 300.mL of
Q86: When an aqueous solution of AgNO<sub>3</sub> is
Q95: Which one of these net ionic