Given the following standard reduction potentials, 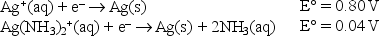 calculate the formation constant of Ag(NH3) 2+ at 25°C.
calculate the formation constant of Ag(NH3) 2+ at 25°C.
Definitions:
Organization's Responsibility
The obligations and duties an organization has towards its stakeholders, including ethical behavior, compliance with laws, and social contributions.
Strictness Error
A bias in performance appraisal where the evaluator tends to judge all employees more harshly than their performance warrants.
High Ratings
Refers to evaluations or assessments that are significantly above average, often indicating superior performance or quality.
Job Design
Job design is the process of specifying job tasks and work arrangements.
Q1: Which one of these metals would normally
Q27: Which one of the following reagents is
Q29: How many coulombs (C)of electrical charge must
Q54: Write the chemical formula of magnetite.
Q78: The half-life of <sup>90</sup>Sr is 29 years.
Q80: Calculate the hydrogen ion concentration in a
Q84: Predict the products of the electrolysis of
Q119: Wilson Inc wishes to use the revaluation
Q122: Acid strength decreases in the series: HNO<sub>3
Q149: Will a 0.1 M solution of NH<sub>4</sub>CN(aq)be