Given the following standard reduction potentials in acid solution 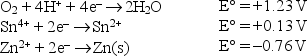 write a balanced equation for a spontaneous reaction which involves the tin and zinc redox couples.
write a balanced equation for a spontaneous reaction which involves the tin and zinc redox couples.
Definitions:
College Grades
The scores or marks given to students based on their academic performance in college coursework.
Scatterplot
A graphical representation using dots to show the relationship between two quantitative variables.
Correlation
A gauge that determines the extent of simultaneous variations between two or more variables.
Lurking Variable
A variable that is not directly measured but affects the variables of interest in a study, potentially confounding the results.
Q2: In the Hall process, _ is reduced
Q24: How is an impairment loss allocated to
Q26: How would you expect the molecule 1,10-phenanthroline
Q26: In p-type semiconductors,<br>A)the semiconductors are "ultra purified"
Q33: Determine the equilibrium constant for the following
Q38: Will Fe(OH)<sub>3</sub> precipitate from a buffer solution
Q39: Which of these reactions represents the
Q50: The total number of electrons in the
Q83: What element is the stable end-product of
Q149: Will a 0.1 M solution of NH<sub>4</sub>CN(aq)be