Consider the following standard reduction potentials in acid solution: 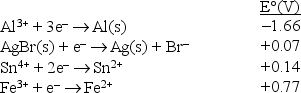 The strongest reducing agent among those shown above is
The strongest reducing agent among those shown above is
Definitions:
Information Management
The process of collecting, storing, managing, and distributing information in an effective and secure manner.
Process Improvement
The proactive task of identifying, analyzing, and improving upon existing business processes within an organization to optimize performance or meet new quotas or standards.
Employee Compensation
The package of wages, salaries, and benefits that employees receive in exchange for their work.
HCR-20
A tool used for assessing the risk of violence in individuals, considering historical, clinical, and risk management factors.
Q3: Predict the number of unpaired electrons in
Q15: Which type of radiation is the least
Q37: Assuming equal concentrations of conjugate base and
Q58: Metallic sodium is obtained commercially from molten
Q59: A current of 2.50 A was
Q75: If the measured voltage of the
Q77: Determine the equilibrium constant K<sub>p</sub> at
Q110: Which of the following yields a basic
Q138: If the pH of tomato juice is
Q156: Calculate the pH of a 1.6 M