Consider the following standard reduction potentials in acid solution: 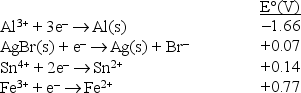 The strongest oxidizing agent among those shown above is
The strongest oxidizing agent among those shown above is
Definitions:
Matching Principle
A concept of accounting in which expenses are matched with the revenue generated during a period by those expenses.
Uncollectible Receivables
Accounts receivable that a company does not expect to collect due to customers’ inability to pay, often written off as a bad debt expense.
Allowance for Doubtful Accounts
A contra-asset account that reduces the total amount of accounts receivable to reflect the estimated portion that may not be collected.
Accounts Receivable
Money owed to a business by its clients or customers for goods or services delivered or used but not yet paid for.
Q13: Calcium metal is produced by electrolysis of<br>A)CaSO<sub>4</sub><br>B)CaF<sub>2</sub><br>C)CaCO<sub>3</sub><br>D)Ca(OH)<sub>2</sub><br>E)CaCl<sub>2</sub>
Q18: Write the formula for the alcohol and
Q19: Which of the structures below corresponds to
Q29: How many coulombs (C)of electrical charge must
Q31: The systematic name of the coordination compound
Q46: Which is correct with respect to the
Q59: Which of these species will act as
Q83: The equilibrium between carbon dioxide gas and
Q107: Which one of the following reactions
Q111: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"