Consider the following standard reduction potentials in acid solution: 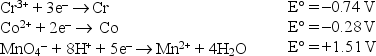 The weakest reducing agent listed above is
The weakest reducing agent listed above is
Definitions:
High Crime Rates
The term refers to a higher than average incidence of criminal activities within a specific area or population over a certain period of time.
Social Influence
The effect of other people on an individual's thoughts, feelings, attitudes, or behaviors.
Scots-Irish Herders
A social and ethnic group of people originating from the Scottish lowlands and the north of Ireland, particularly noted for their historical involvement in herding and agriculture.
High Violence Rates
Elevated frequencies of violent acts or behaviors within a specified population or area.
Q8: The intermolecular force between bases on the
Q13: In the coordination compound [Co(en)<sub>2</sub>Cl<sub>2</sub>]Cl, the coordination
Q14: Which one of these species is an
Q23: Which of these molecules is unsaturated?<br>A)CH<sub>4</sub><br>B)C<sub>2</sub>H<sub>6</sub><br>C)C<sub>4</sub>H<sub>6</sub><br>D)C<sub>5</sub>H<sub>12</sub><br>E)C<sub>6</sub>H<sub>14</sub>
Q30: The two molecules represented below are examples
Q31: Decay of tin-110 by electron capture yields<br>A)tin-109.<br>B)cadmium-106.<br>C)indium-110.<br>D)antimony-110.<br>E)tellurium-114.
Q88: What is the concentration of H<sup>+</sup> in
Q89: Determine how much energy is released
Q93: Will a precipitate of AgCl form when
Q105: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="Balance the